
Let’s take a look at a classic example on how relative atomic mass is being calculated.Ĭhlorine exists as chlorine-35 (% abundance of 75%) and chlorine-37 (% abundance of 25%).
The Periodic Table used by GCE A-Level H2 Chemistry students in Singapore The following Periodic Table is used by GCE O-Level Chemistry students.
#ATOMIC MASS NUMBER FORMULA CODE#
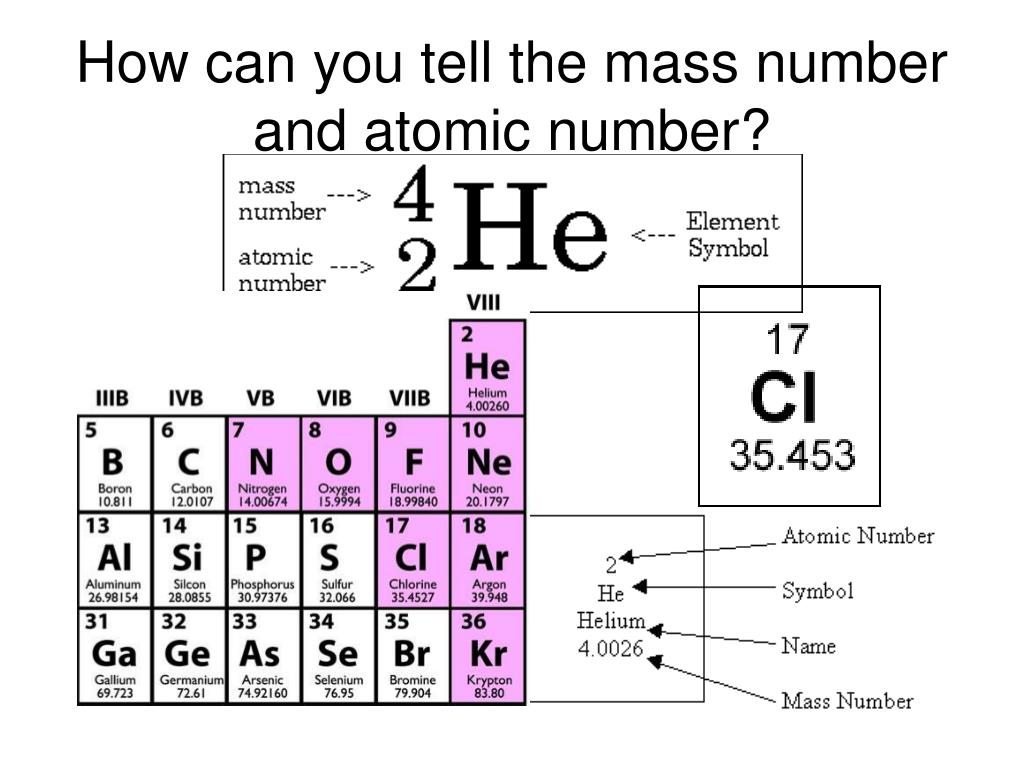
So, relative atomic mass is basically an average value after considering all the different isotopes of the element.įor GCE O-Level Pure Chemistry students (syllabus code 6092) in Singapore, the Periodic Table is not as precise as the one used by the GCE A-Level H2 Chemistry students (syllabus code 9729). The relative atomic masses that you see in the Periodic Table are calculated based on the relative percentage abundance of the isotopes. These elements exist as a mixture of isotopes (which have different mass numbers). If you refer to the Periodic Table, you will notice that the relative atomic masses of some elements are not whole numbers. Relative atomic mass is a ratio and therefore has no unit. The symbol for relative atomic mass is Ar. To be more precise, the masses of all elements (in terms of atom) listed in The Periodic Table are always compared to 1/12 of the mass of one atom of carbon-12. The relative atomic mass of an atom is the average mass of one atom of that element compared to 1/12 of the mass of one carbon-12 atom.īasically, it is not practical for scientists to use actual masses of atoms in scientific calculations since atoms have very small masses.Īs such, scientists compare masses of different atoms with reference to the carbon-12 atom (which is an isotope of carbon). Let’s take a look at them one at a time…. In order to differentiate them (as well as see how they are connected to each other), we have to know their definitions first. Many students are confused when it comes to the difference between the following terms commonly used in Mole Concept & Chemical Calculations, namely:


 0 kommentar(er)
0 kommentar(er)
